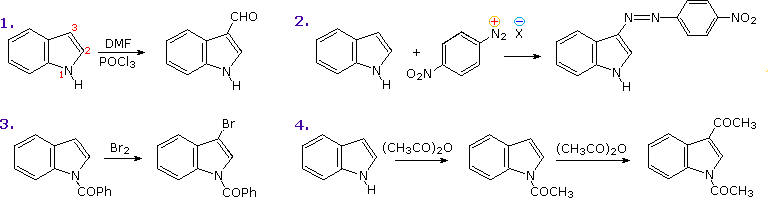
Molecules | Free Full-Text | Functionalised Oximes: Emergent Precursors for Carbon-, Nitrogen- and Oxygen-Centred Radicals

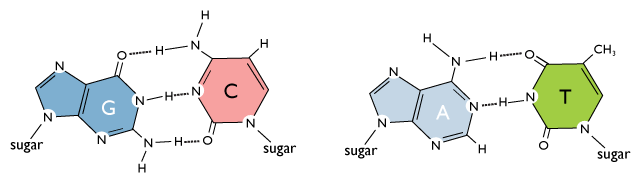
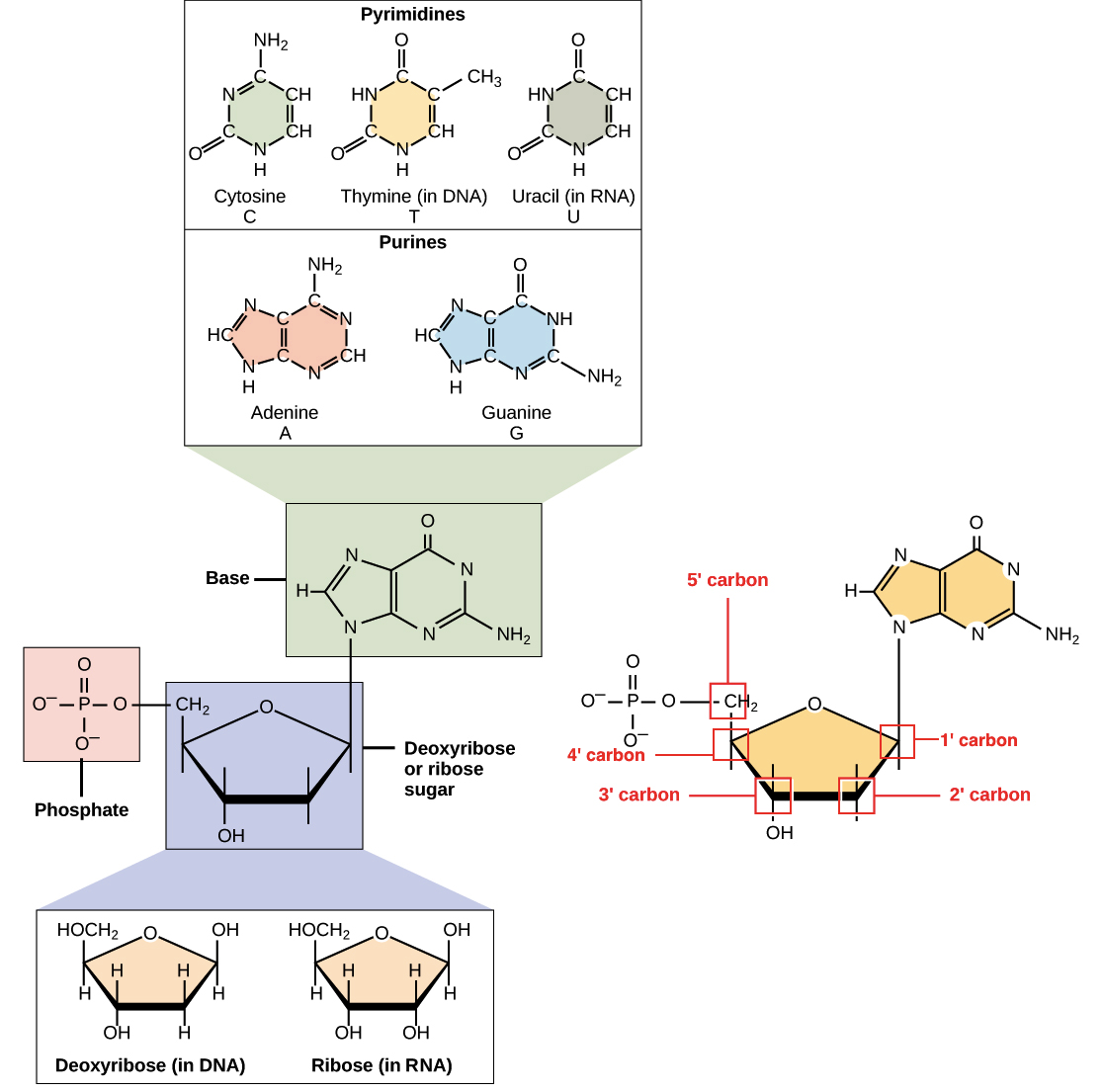
Molecular BiochemistryBioc.432 Lab 1: Introduction to nucleic acids (Structural properties) - ppt download
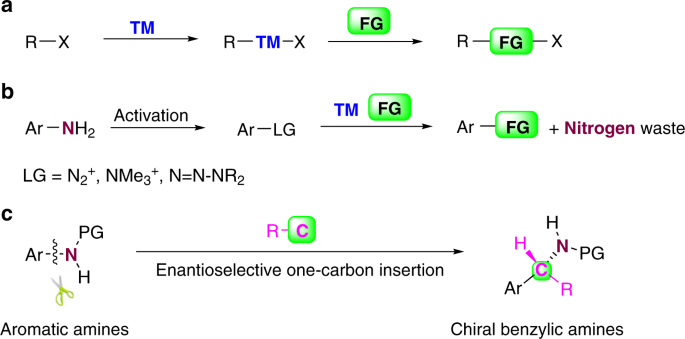
Conversion of anilines to chiral benzylic amines via formal one-carbon insertion into aromatic C–N bonds | Nature Communications
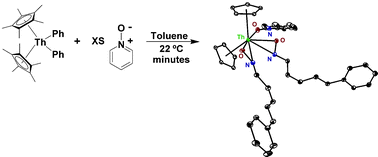
Carbon–nitrogen bond cleavage in pyridine ring systems mediated by organometallic thorium(iv) complexes - Chemical Communications (RSC Publishing)

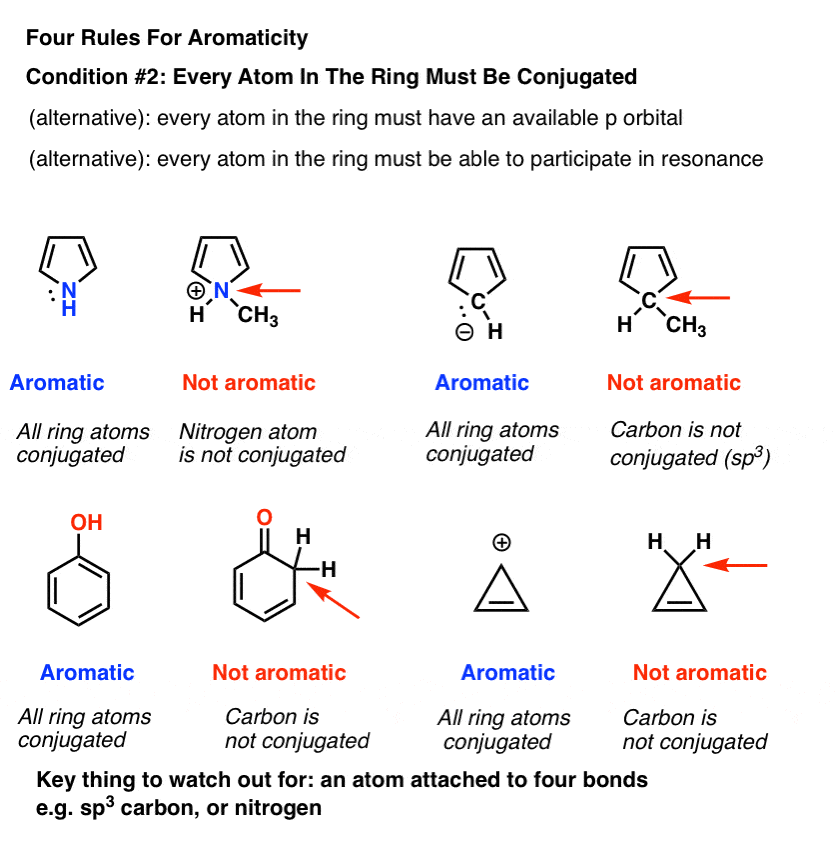
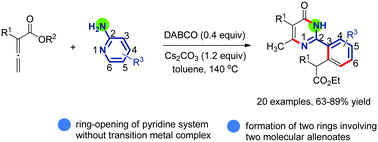
Nitrogen heterocycles are carbon organic rings containing nitrogen. Nitrogen heterocycles can be formed from aliphatic nitro… | Nitrogen, Amino acids, Organic rings

DNA, Mitosis, and Meiosis Learning Target Objectives: I can… Describe the structure of, base pairing, and roles (importance) of both DNA and RNA. Explain. - ppt download

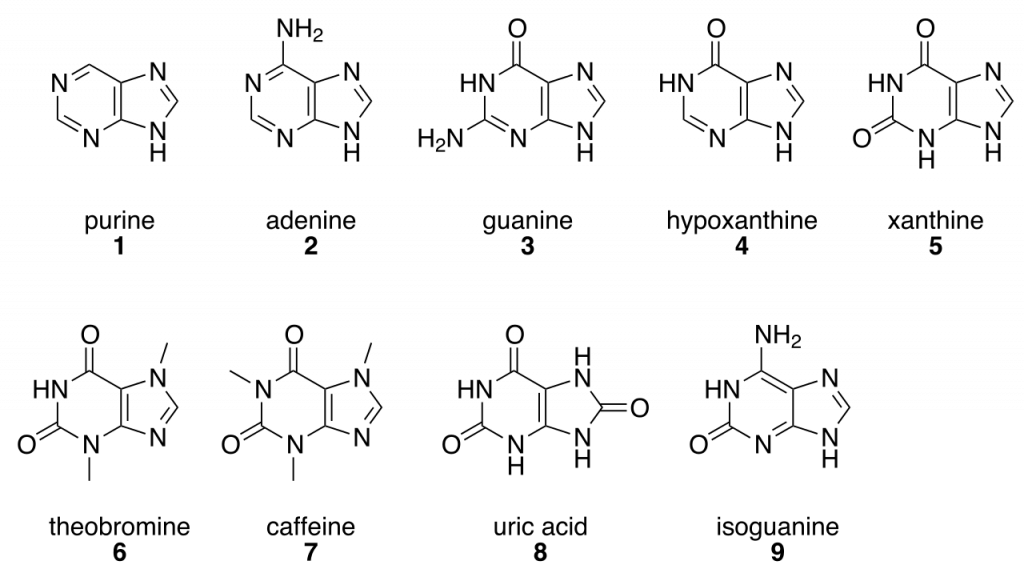
Carbon and nitrogen labelling of pyrimidine and purine rings. (a) When... | Download Scientific Diagram

Phosphorylation of Arabidopsis Ubiquitin Ligase ATL31 Is Critical for Plant Carbon/Nitrogen Nutrient Balance Response and Controls the Stability of 14-3-3 Proteins* - Journal of Biological Chemistry

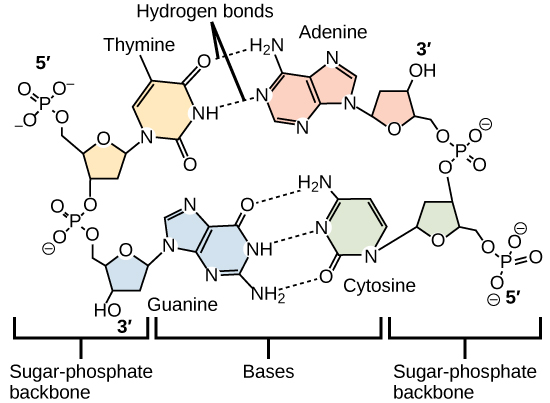
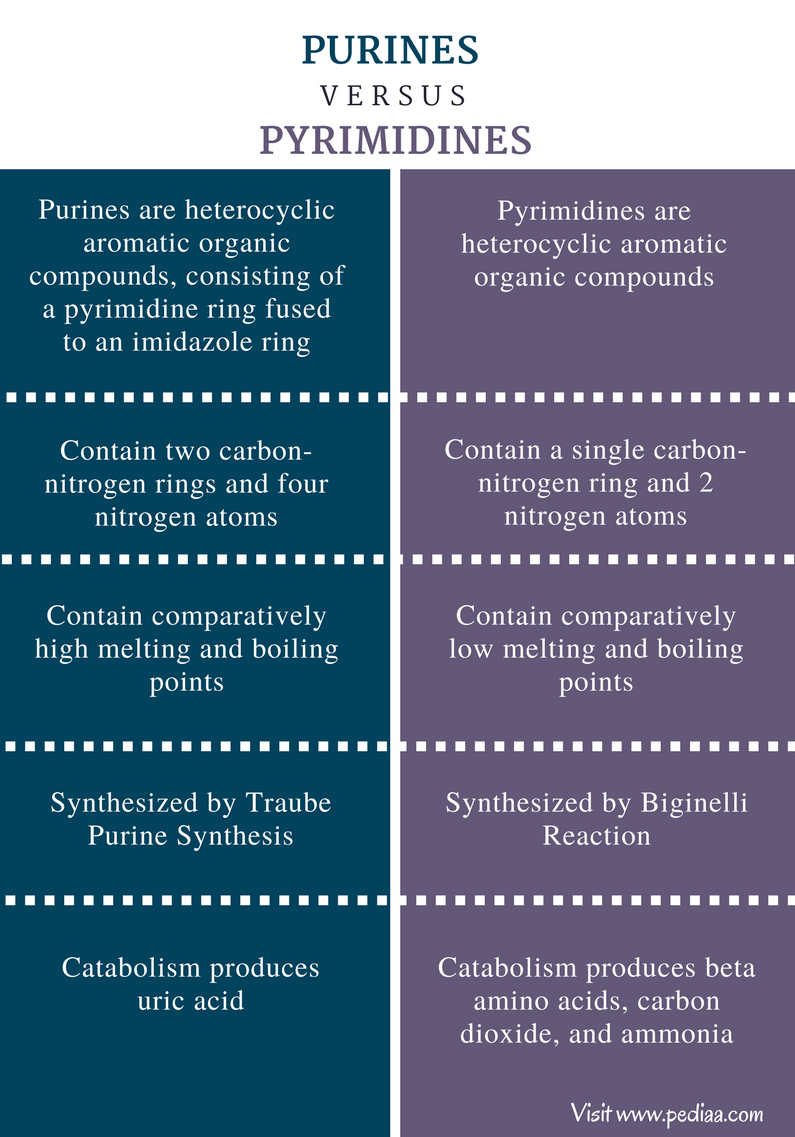
:max_bytes(150000):strip_icc()/purine-and-pyrimidine-nitrogenous-bases---skeletal-chemical-formulas-475632152-d34de0fec4e14f108a3e32901a1386c8.jpg)